VEXTEC’s Vice President of Business Development, Dr. Sanjeev Kulkarni is an invited speaker at the ASME Advanced Design and Manufacturing Impact Forum 2014, August 17-20, 2014 at the Buffalo-Niagara Convention Center in Buffalo, NY. He will be presenting a talk entitled “Medical Devices & Life Sciences: Innovative Medical Device Prototyping – 3D Printing and Biocompatibility”. The presentation is one of five talks at the conference that will we be webcast live.
Dr. Kulkarni will be discussing additive manufacturing (AM) which is the process of taking a digital representation of a part or component and directly manufacturing the resulting product using a three-dimensional fabrication technique. AM is still in the nescient stages, and as with any new technology, it brings with it the burden of certification, especially in the safety conscious medical device community. While common alloys are being developed, they are considered a new “product form” and therefore require all of the scrutiny and qualification as a new material. The AM process contains many variables that can be varied and there are multiple machines that have very similar elements, but produce statistically significant variations of product. As such, it is critical to define the optimal process and, once established, properly characterize the material system in all structural categories sufficient for analysis and establishment of margins. Traditional test and evaluation methods for material certification for high safety applications can take decades and cost millions of dollars.
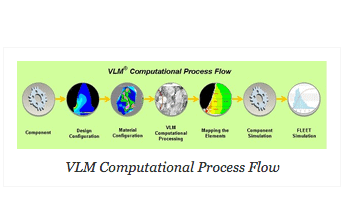
VEXTEC has integrated computational material engineering (ICME) software called Virtual Life Management® (VLM®)that can be used to directly simulate AM products and provide rapid accept/reject criteria early in the design stage and enable robust design philosophies to be incorporated. The method significantly lowers unit costs of AM products by reducing the high barrier to entry caused by the current certification test requirements. Manufacturers will be able to determine if new, less costly AM processes (or vendors) will provide a reliable product. A computational material simulation software that offers the ability to complete most of the component validation process with limited testing, will create a change in material science much the way FEA changed structural design.
Program topics include Design, Advanced Manufacturing, Additive Manufacturing, Aerospace, Automotive, Life Sciences & Medical Devices, Computer Aided Engineering and Robotics.




Leave A Comment